
CAR T-Cell Therapy Approved for Some Children and Young Adults with Leukemia
September 11, 2017, by NCI Staff
On August 30, the Food and Drug Administration (FDA) approved a type of immunotherapy called CAR T-cell therapy for certain children and young adults with a form of acute lymphoblastic leukemia(ALL). The treatment, tisagenlecleucel (Kymriah™), is the first CAR T-cell therapy to receive FDA approval.
Acute lymphoblastic leukemia is the most common cancer among children in the United States. Intensive chemotherapy cures more than 80% of children with ALL that arises in B cells, which is the predominant type of pediatric ALL. But there are few treatment options for patients whose cancers do not respond to treatment or go into remission and later relapse.
FDA approved tisagenlecleucel, which is manufactured by Novartis, for patients up to 25 years of age with B-cell ALL that does not respond to treatment or has relapsed two or more times.
The treatment is customized for each patient using the patient’s T cells, which are genetically modified to enhance their ability to recognize and attack cancer cells. The process takes about 22 days and involves isolating the cells from the patient, genetically engineering and expanding them in the laboratory, and returning them to the center where the patient is treated.
High Remission Rates with Tisagenlecleucel
The approval of tisagenlecleucel was based on a multicenter clinical trial involving 63 children and young adults with B-cell ALL that had relapsed or resisted treatment. Within three months of treatment, the overall remission rate was 83%.
The treatment was initially tested at the University of Pennsylvania in 2010 among adults with advanced chronic lymphocytic leukemia (CLL). In 2012, the Children’s Hospital of Philadelphia (CHOP) became the first institution to test the treatment in children with ALL. Since then, the treatment has been evaluated at 13 US medical centers as well as some in other countries.
“We’re seeing overall remission rates over 80 percent, which is a remarkable improvement upon previous treatment success rates,” Stephan Grupp, M.D., Ph.D., who led the trial at CHOP, said in a statement.
Another CAR T cell therapy, produced by Kite Pharma, is expected to be approved later this year for the treatment of some patients with lymphomas. With the success seen in blood cancers, researchers at NCI and other organizations are busy developing ways to expand the use of CAR T-cell therapies.
“We hope the momentum behind the technology builds as we continue to investigate the abilities of personalized cellular therapeutics in blood cancers and solid tumors to help patients with many other types of cancer,” Carl June, M.D., of the University of Pennsylvania Abramson Cancer Center, who led the early development of tisagenlecleucel, said in a statement.
A Personalized Therapy
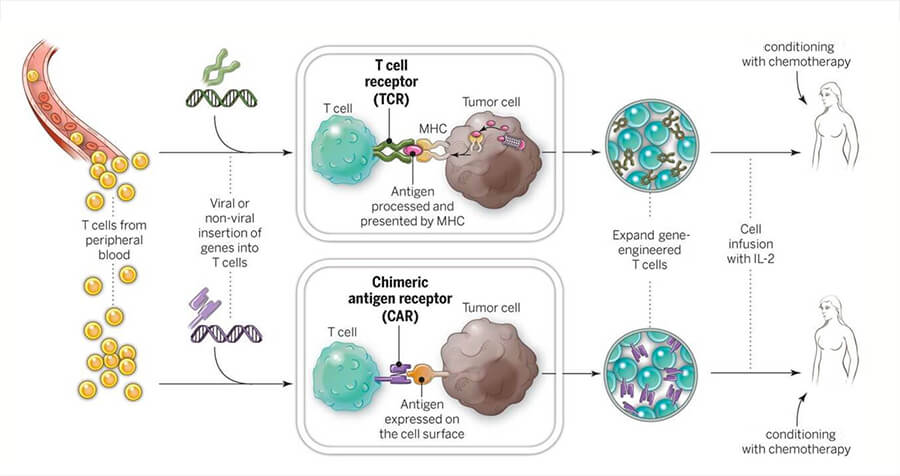
The first step in creating tisagenlecleucel is to isolate T cells from a patient’s blood. These are shipped to a cell-processing plant. There, the cells are genetically engineered so that they will produce a protein called a chimeric antigen receptor, or CAR, which allows the cells to attach to a protein on tumor cells called CD19. The modified cells are then grown, or expanded, into hundreds of millions of cells and shipped back to the patient’s physician.
Finally, after patients undergo a "lymphodepleting" chemotherapy, they receive the infusion of the engineered immune cells. If the treatment works as intended, the CAR T cells will continue to multiply, increasing the therapy’s potential for eradicating the cancer.
Managing CAR T-Cell Side Effects
Some patients in the trial experienced serious side effects associated with the treatment, and Novartis will include a “boxed warning” to alert physicians to the potential health risks.
The infusion of CAR T cells can lead to inflammatory side effects—known collectively as cytokine release syndrome (CRS)—that range from high fevers and mild flu-like symptoms to life-threatening drops in blood pressure, vascular leakage, and kidney problems. In addition, neurological events, such as confusion and seizures, are also possible and may be life threatening.
In the trial that led to FDA approval, CRS was managed using the arthritis drug tocilizumab (Actemra).
“Actemra seems to blunt the symptoms of CRS in the vast majority of patients,” said Christian Capitini, M.D., of the University of Wisconsin (UW) School of Medicine and Public Health, who treated participants in the clinical trial at the American Family Children’s Hospital at UW.
FDA has now expanded the approval of tocilizumab to treat severe or life-threatening CRS in patients over the age of 2. In clinical trials involving CAR T cells, 69% of the patients who received one or two doses of tocilizumab experienced a complete resolution of CRS within two weeks, according to FDA.
“The neurological side effects in patients seem to resolve on their own,” Dr. Capitini said. “But it’s something we have to watch for.”
FDA is requiring that institutions offering tisagenlecleucel be certified to demonstrate that they have the expertise to treat patients and monitor them for potential side effects. Certification will involve, for instance, training staff to recognize and manage CRS and neurological events.
Answering Questions, Next Steps for CAR T Cells
Follow-up care for patients with ALL who receive CAR T cells includes monthly outpatient immunoglobulin infusions to prevent infections. Patients have an increased risk of infections because CD19 is present on normal B cells (as well as cancerous ones), and these normal cells are also destroyed by CAR T-cell therapy.
“Many questions must be addressed before we can herald immunotherapeutic approaches to cancer an unqualified success,” wrote NIH Director Francis Collins, M.D., Ph.D., in a blog post about the FDA approval. “There are still too many severe reactions, too many non-responses or relapses, and, potentially, a very high price tag for their widespread use, which will be truly challenging to scale up.”
Nonetheless, Dr. Collins added, “We’re off to a promising start.”
Dr. Capitini agreed, noting that many of the patients in the trial who experienced remission had run out of treatment options before starting the CAR T-cell therapy.
“The FDA approval is a milestone for the field, but this is only the beginning,” he said. “We’re going to see CAR T cells tested in different types of cancers. And we are likely to see these therapies being tested earlier in the treatment process, rather than when patients have run out of treatment options.”
NCI at Forefront of CAR T-Cell and Other Cellular Immunotherapy
For nearly 2 decades, NCI researchers have led the effort to advance cellular therapies for cancer—research that laid the groundwork for the approval of tisagenlecleucel. This research includes developing and testing CAR T-cell therapies and two other forms of adoptive cell transfer: T-cell receptor (TCR) and tumor infiltrating lymphocyte (TIL) therapies.
NCI researchers are currently conducting many clinical trials testing CAR T-cell, TCR, and TIL therapies in children and adults with cancer. Some of these trials include:
- TCR therapy in patients with HPV-related cancers
- CAR T cells in patients with CD22-positive leukemias or lymphomas
- TILs in patients with advanced non-small cell lung cancer
- CAR T cells in CD30-positive lymphomas
“The approval of the first CAR T-cell therapy really is a landmark moment for cancer immunotherapy,” said William Dahut, M.D., scientific director for clinical research in NCI’s Center for Cancer Research. “Our focus at NCI is on improving the efficacy of these cellular immunotherapies and finding ways to make them applicable to as many cancer types as possible.”






















.png)









No hay comentarios:
Publicar un comentario