

Unexpected’ Vulnerability Creates Treatment Opportunity in Aggressive Type of Lung Cancer
October 27, 2016 by NCI Staff
Researchers have identified a potentially critical weakness in lung cancers that have mutations in the KRAS gene, a cancer-promoting genetic alteration that has proven nearly impossible to target therapeutically. Moreover, the research team showed that a drug already being tested against other types of cancer could successfully exploit this vulnerability in KRAS mutant lung cancer cell lines and mouse models of lung cancer.
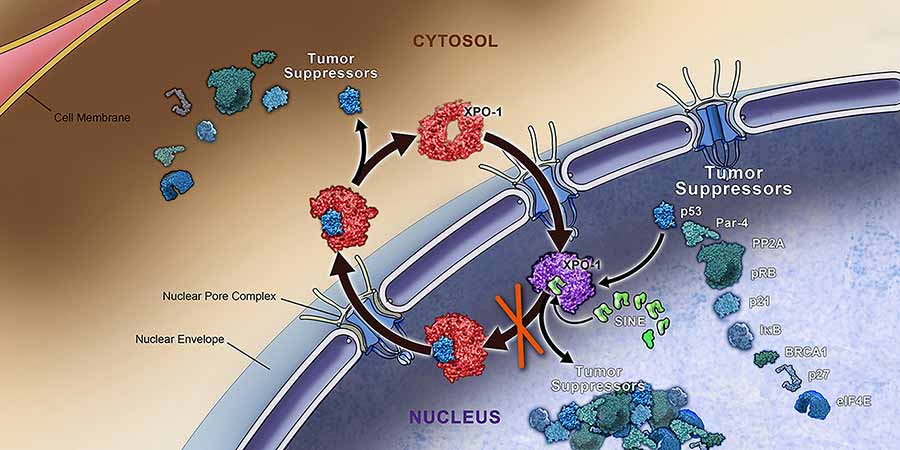
The weakness lies in the dependency of KRASmutant lung cancer cells on a protein, called XPO1, that helps to shuttle molecules involved in important cellular functions from the cell nucleusinto the surrounding cytoplasm. The XPO1 inhibitor shrank tumors in several different mouse models of non-small cell lung cancer (NSCLC)—the most common form of lung cancer—with KRAS mutations.
The study results, wrote lead investigator Jimi Kim, Ph.D., of the University of Texas (UT) Southwestern, and her colleagues, suggest that “XPO1 inhibitors are a promising therapeutic strategy for a considerable cohort of patients with lung cancer.”
The study was published October 6 in Nature.
In Search of Synthetic Lethality
The RAS family of genes—which, in addition to KRAS, includes NRAS and HRAS—are among the most heavily studied oncogenes.
But efforts to develop therapies that directly target mutant Ras proteins in tumors have been stymied by several factors, including their complex biology and unique structural and biochemical properties, explained Ji Luo, Ph.D., of the Laboratory of Cancer Biology and Genetics in NCI’s Center for Cancer Research.
Mutations in KRAS are present in tumors of approximately 25% of patients with NSCLC. Patients whose tumors have these mutations typically have a poor prognosis, and even if their cancer responds to initial treatment with chemotherapy, the disease almost invariably returns. So finding safe and effective ways to treat KRAS-mutant tumors in patients with NSCLC is a high priority.
One way researchers are attempting to overcome the difficulty of directly targeting RAS proteins is to search for an end-around: identifying and targeting molecular partners or signaling pathways on which they are highly dependent to carry out their tumor-promoting functions. Take out the partner, the thinking goes, and you create a chain reaction that leads to cancer cell death—a concept known as “synthetic lethality.”
The UT Southwestern researchers took this synthetic lethality approach in their study. Based on a comprehensive genomic analysis of more than 100 high-quality NSCLC cell lines, the research team selected 12 lines that best represented the genomic makeup of NSCLC, explained the study’s senior author, Michael White, Ph.D. They then performed a synthetic lethal “screen” on these 12 cell lines, systematically turning off, or silencing, genes to identify those genes whose inactivation led to cell death in cancer cell lines that had KRASmutations (but not those with unmutated KRAS).
From these experiments, the researchers discovered that a group of genes involved in transporting molecules out of the cell nucleus was necessary for survival of KRAS-mutated NSCLC cells.
Starting with XPO1
The reliance of tumors with KRAS mutations on these so-called nuclear export proteins led the team to XPO1. This was not because the screen identified XP01 as being more important than other nuclear export proteins, explained study co-author Pier Paolo Scaglioni, M.D., but because, by “a lucky circumstance,” an XPO1-targeted drug, KPT-330, had already been developed and is being tested as a treatment for several cancers.
The researchers found that the drug (as well as an earlier version of it) killed most KRAS-mutant NSCLC cancer cell lines and shrank tumors in different types of mouse models of KRAS-mutant NSCLC, including two models that closely replicate how the cancer behaves in humans.
The reliance of mutant KRAS on the nuclear export pathway is “very unexpected,” said Dr. Luo, who was not involved in the study. The finding, he continued, “illustrates that there might be additional unanticipated targets that can be exploited in KRAS mutant cancers.”
The research team also identified why targeting XPO1 may cause cancer cells with KRASmutations to die. Blocking XPO1’s activity, they showed, disrupted a signaling pathway controlled by a transcription factor, NFκB, that affects the activity of many genes involved in promoting cell survival. By interfering with the NFκB-supplied survival signals, Dr. Scaglioni said, KRAS mutant cancer cells soon die.
But not all of the mutant KRAS NSCLC cell lines and animal models responded to treatment with KPT-330. The UT Southwestern team was able to trace this resistance to a common factor: a mutation in the FSTL5 gene. This mutation, they found, leads to the overexpression of the YAP1 protein, another transcription factor that recent studies have suggested can fuel tumor growth. Blocking YAP1 in KRAS-mutant NSCLC cell lines, they showed, could overcome resistance to the XPO1 inhibitor.
Identifying mechanisms of resistance as early as possible in the development of new targeted therapies is important, Dr. Luo said. He cited the example of FDA-approved drugs that target tumors with mutations in the BRAF and EGFR genes. Although many patients initially respond well to these drugs, resistance almost invariably sets in.
So having data on resistance “can help us to stay one step ahead of the tumor,” he continued. “It can help identify potential strategies to target these resistance mechanisms, or prevent resistance from emerging in the first place.”
Human Trials and Future Directions
Karyopharm Therapeutics, which developed KPT-330 (also known as Selinexor), is already testing the drug in a number of early-stage clinical trials in patients with blood cancers and solid tumors.
Based on the results from this new study in cell lines and mouse models, the company plans to launch an early-stage trial early next year testing KPT-330 in combination with the chemotherapy drug docetaxel in patients with advanced NSCLC that has KRAS mutations.
Although these new results are important and researchers are starting to make some headway in developing methods for directly targeting RAS, Dr. Scaglioni said, research to identify synthetic lethal partners for this common oncogene will continue to be a priority.
Dr. Luo agreed, noting that a synthetic lethal approach has important advantages. By casting a wider net, he continued, researchers may identify more processes involved in promoting cancer “that are unexpected and potentially druggable.”























.png)









No hay comentarios:
Publicar un comentario